
Not All ISO 27001 Certificates are Equal: UKAS vs Non UKAS Accreditation
Cordi Mahony•Feb 11, 2026
Not All ISO 27001 Certificates are Equal: UKAS vs Non UKAS AccreditationDigital Clinical Safety for Manufacturers
The DCB0129 is a mandated requirement for manufacturers of health IT systems to ensure digital health technologies are safe to use in the NHS. Ensure safe, efficient, and cost-effective deployment of health IT systems by meeting your DCB0129 requirements
Request a demoNHS Digital Clinical Safety for Manufacturers
We are trusted by
We can assist you with all aspects of DCB0129 Clinical Risk Management including:
Create Clinical Safety Case Reports and Clinical Risk Management Plans. Generate documents from templates; automate document completion; set owners, due dates and reminders; collaborate across teams.
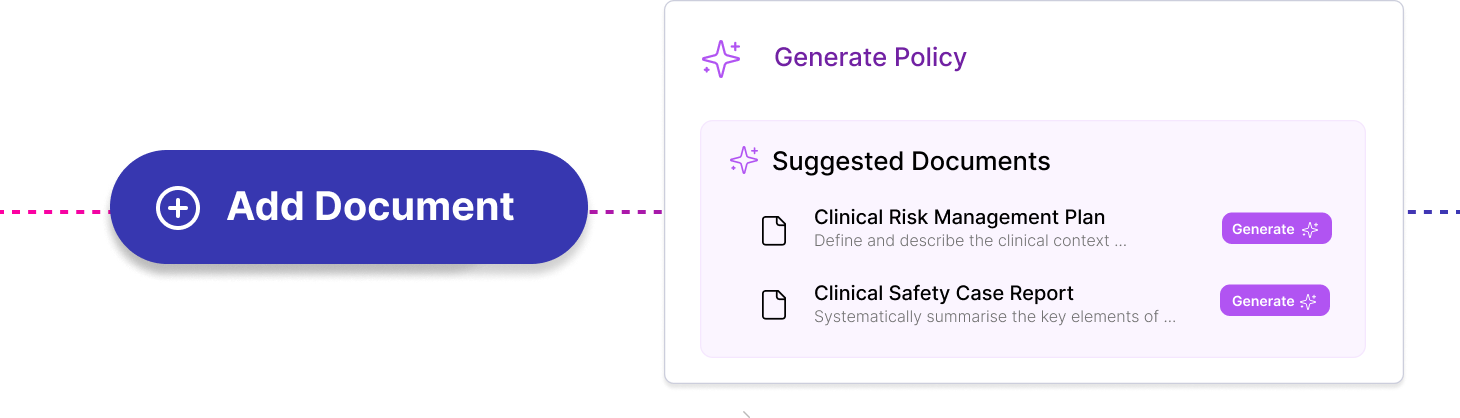
Work across multiple complex projects. Avoid duplication of work by linking hazards, causes and controls across projects.
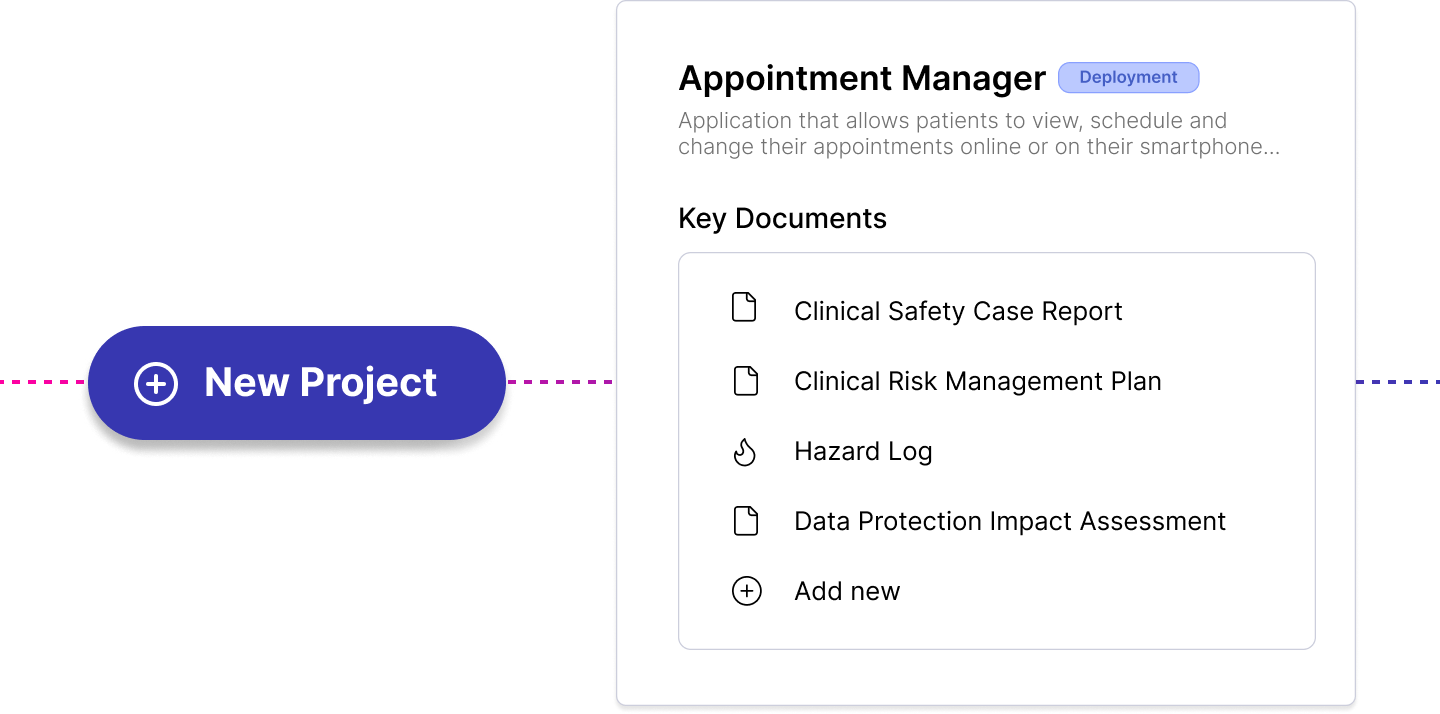
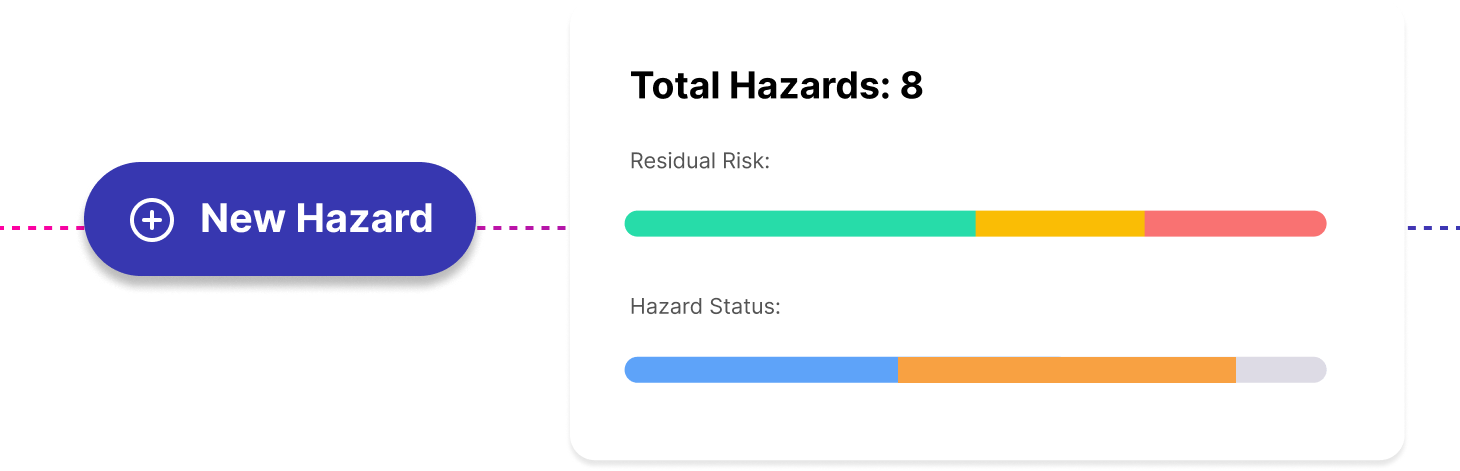
Best-in-class hazard log designed by Clinical Safety Officers and specialised for DCB0129. Manage hazards, causes, controls and evidence across multiple projects. Referenced to the implementation guidance throughout.
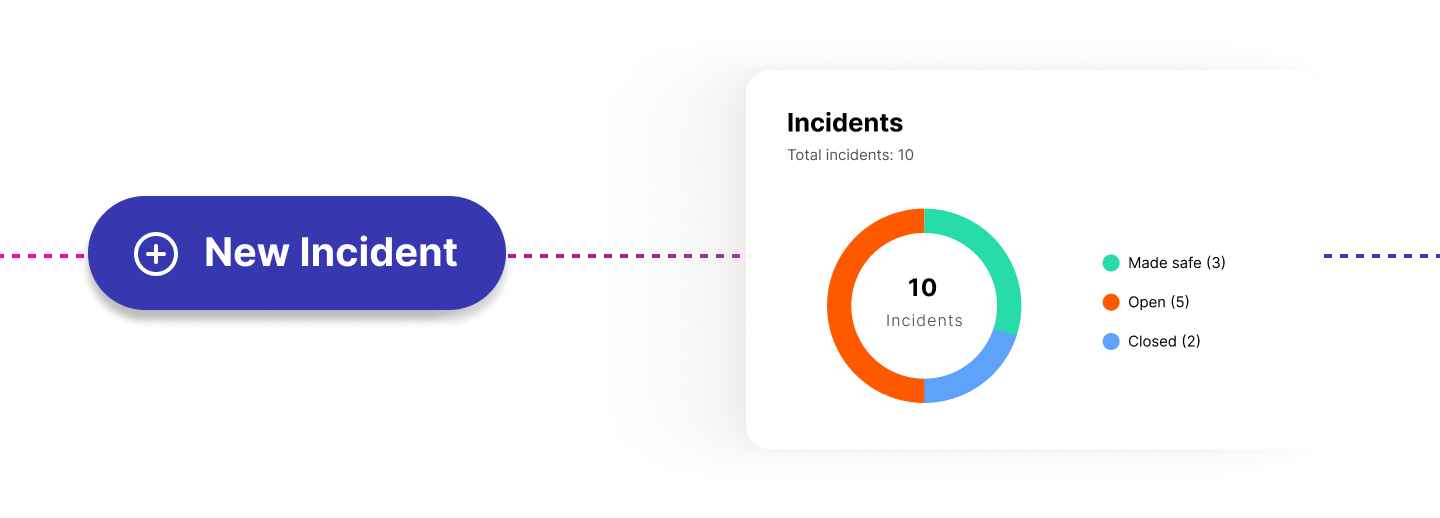
Manage clinical incidents in line with the implementation guidance. Assign owners and link across hazards and controls.
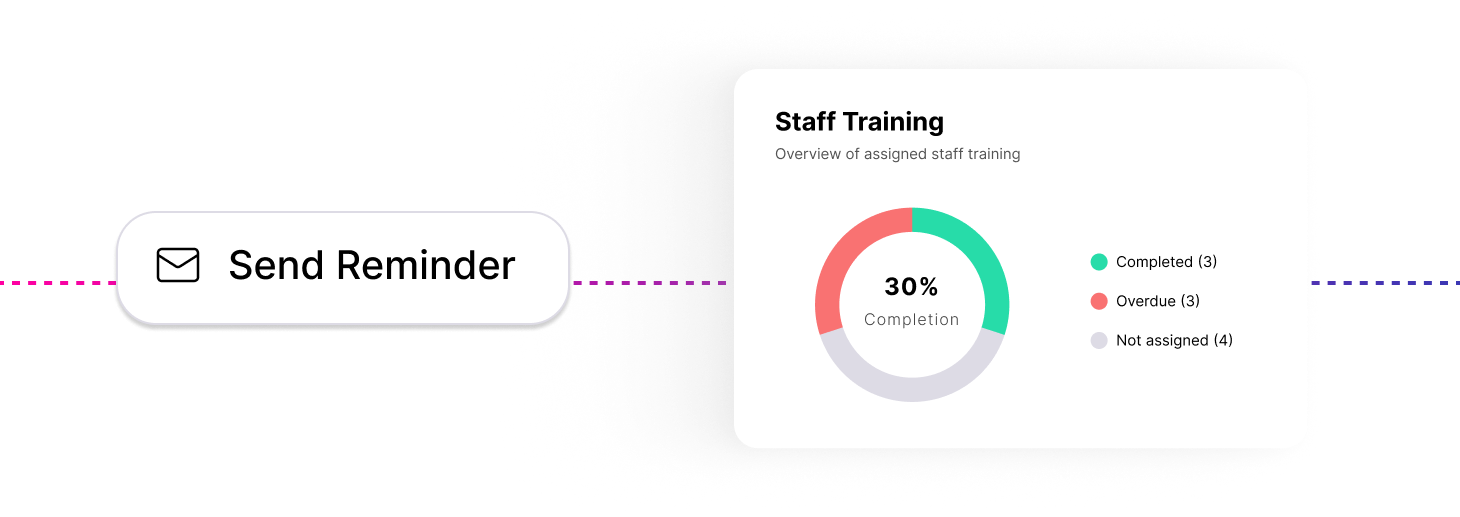
In-built clinical safety training modules, combined with automated tracking and reminders to ensure compliance.
Expert support from our team of Clinical Safety Officers ranging from ad-hoc advice to full managed named external Clinical Safety Officer consultancy.
Use intelligent automation and AI to avoid duplication of work, easily meeting NHS DTAC requirements. Easily share transferred controls with NHS teams and collaborate across clinical safety cases.

Get in touch if we haven’t answered your question below, we are always happy to help!
DCB0129 is a mandatory UK clinical risk management standard that all manufacturers of digital health technologies and health IT systems must comply with.
Yes, all suppliers of digital health technologies or health IT systems must comply with DCB0129. NHS organisations will require evidence of compliance before procurement or implementation.
DCB0129 and DCB0160 are overlapping clinical safety standards. DCB0129 applies to manufacturers or suppliers of digital health technologies or health IT systems. DCB0160 applies to the NHS organisations implementing them.
A Clinical Safety Officer (CSO) is a clinician, currently registered with a professional body, who is appropriately trained in clinical safety. In order to become compliant with DCB0129, you must have a nominated individual to act as your CSO. They can be internal or external - we provide expert external CSOs as required.
A Clinical Safety Officer must:
We work with external CSOs who are experienced and confident using our platform to ensure you are compliant as quickly as possible.
Compliance is complex, but our AI tools simplify it. Discover features to stay ahead of regulations.
Kelly Klifa
CEO at Heim
Assuric has been transformative for Heim as we looked to achieve DCB0129 and DTAC compliance. The platform is easy to use, and the AI tools and automated reminders make previously dreaded compliance tasks a breeze. Paul and Matt supported us every step of the way.
Katie Baker
Director UK & Ireland at Tandem
Assuric has been fantastic in helping us quickly and safely navigate regulatory compliance in the UK. From completing Cybersecurity requirements to DSPT, DCB0129, and DTAC, the team was supportive, extremely knowledgeable, and the platform made everything quick and straightforward. A separate regulatory company we consulted at the beginning even remarked on how quickly we achieved compliance!
Maks Kozarzewski
COO at VitVio
We couldn't speak highly enough of both the Assuric team and the platform itself, which is incredibly easy to use, and with the skill and hardworking nature of the Assuric team. They've been a key component in accelerating our progress and deployments!
Maja Mazur
CEO at Healthnix
Assuric has been such a blessing in getting our DTAC and GDPR compliance done - completing all the documentation and deciding what needs to be done whilst running the business is very hard, but the team really helped us through that. The platform is easy to use, helps keep track of things and it even allows us to coordinate all the team training easily. Highly recommend them!
Dean Mawson
Clinical Director at DPM
Assuric streamlines the process of achieving and maintaining compliance with DCB0129 standards for digital health technologies. The user-friendly interface simplifies collaboration across multidisciplinary teams, while the built-in templates and workflows save significant time and effort during compliance projects. Assuric’s ability to centralise documentation and provide real-time visibility into project progress is particularly beneficial for Clinical Safety Officers and digital project teams, enhancing both efficiency and assurance.

Cordi Mahony•Feb 11, 2026
Not All ISO 27001 Certificates are Equal: UKAS vs Non UKAS Accreditation
As UK healthtechs expand into the US and sell to enterprise customers, SOC 2 often becomes a key requirement. This guide explains what SOC 2 is, who it’s for, the difference between Type I and Type II, and how it helps unlock enterprise deals.
Cordi Mahony•Feb 04, 2026
SOC 2 for Healthtech: Unlocking Opportunities in the USGoodbye manual processes, hello automation. Let Assuric manage compliance and security, so you can focus on growth.
