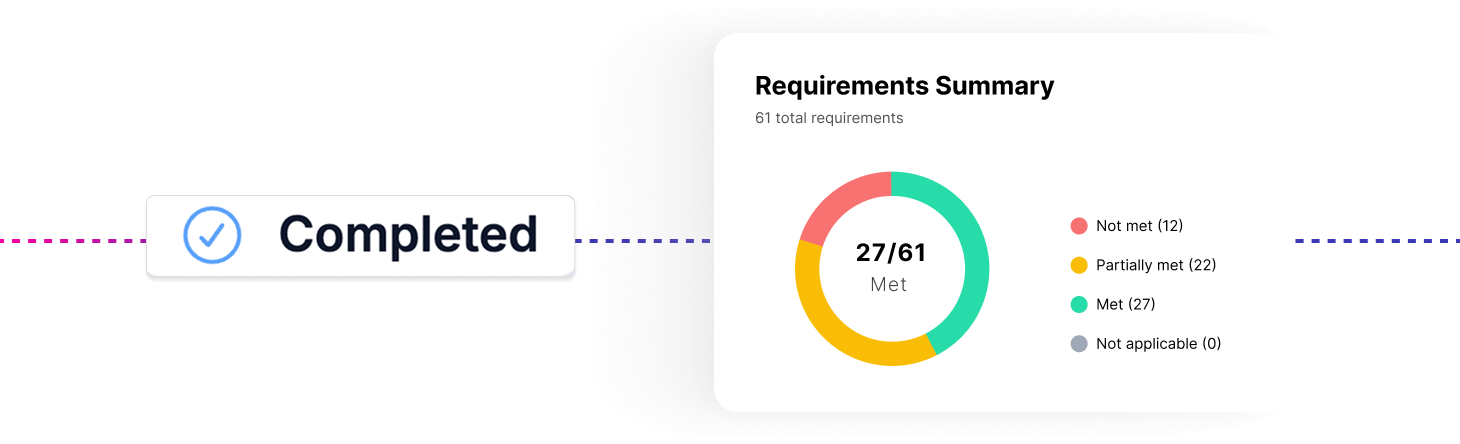
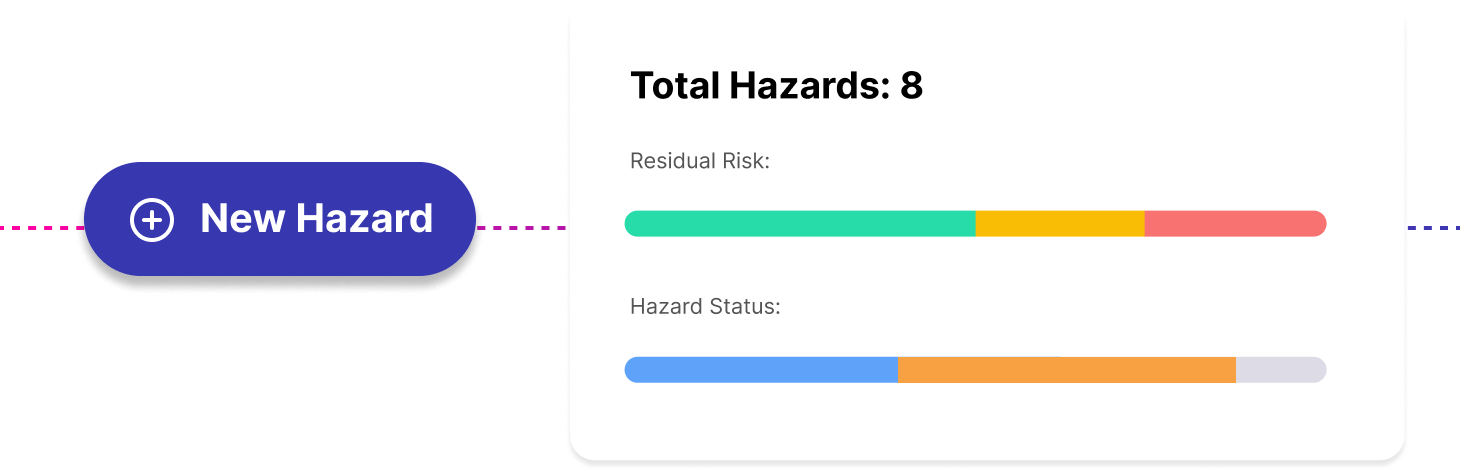
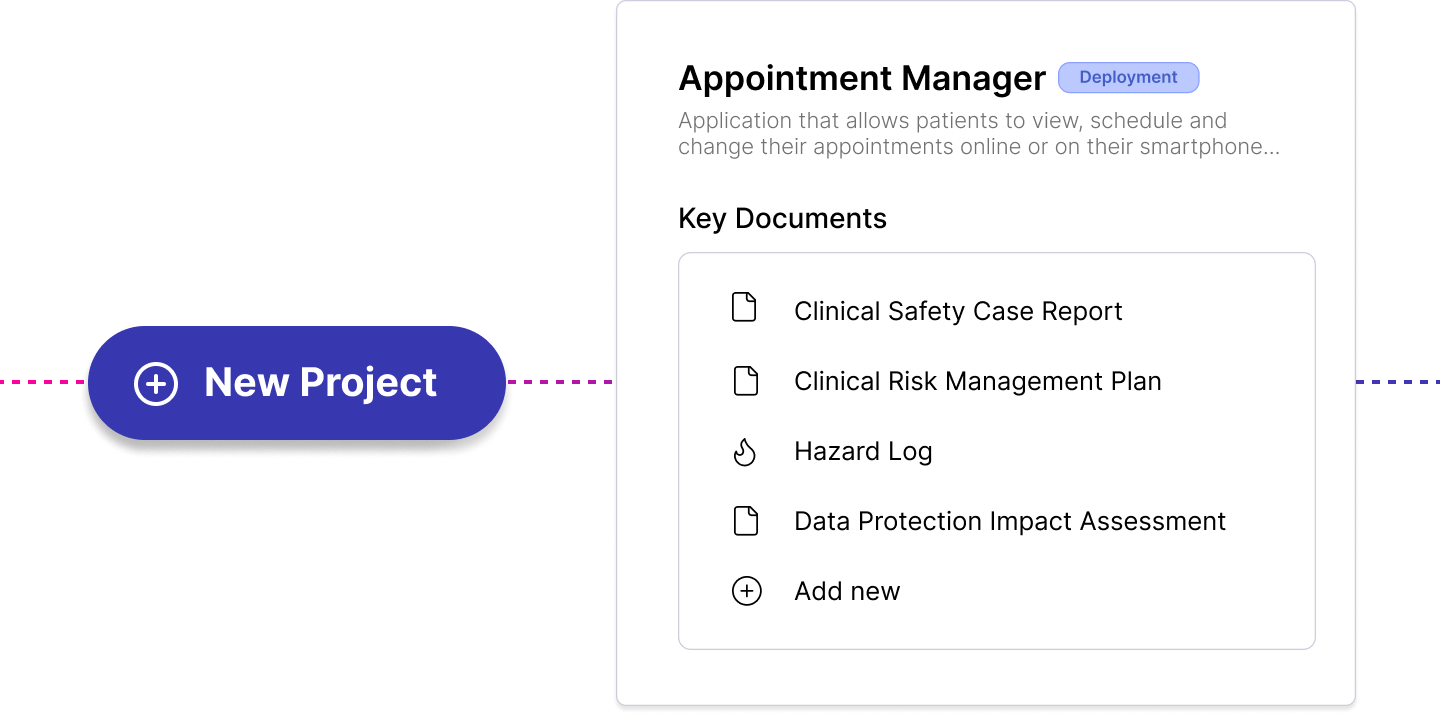
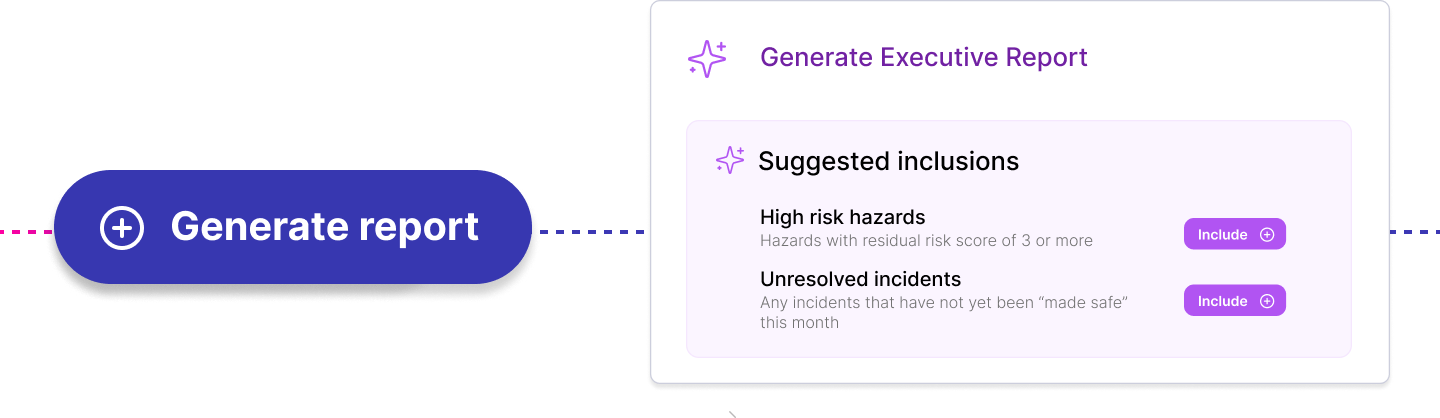
DTAC 2.0: Key Changes and What Healthtech Teams Need to Know
Key changes to the updated DTAC in February 2026, including the changes to the DTAC form and updated Scope
Cordi Mahony•Feb 26, 2026
DTAC 2.0: Key Changes and What Healthtech Teams Need to Know